Featured
- Get link
- X
- Other Apps
Fick's First Law Calculator
Fick's First Law Calculator. A diffusion process that obeys fick's laws is called normal or fickian diffusion; They were derived by adolf fick in.
Application of fick’s law biological application: Diffusion is the net movement of a substance (e.g., an atom, ion or molecule) from a region of high concentration to a region of low concentration. They can be used to solve for the diffusion coefficient, d.fick's first law can be used to derive his second law which in turn is identical to the diffusion equation.
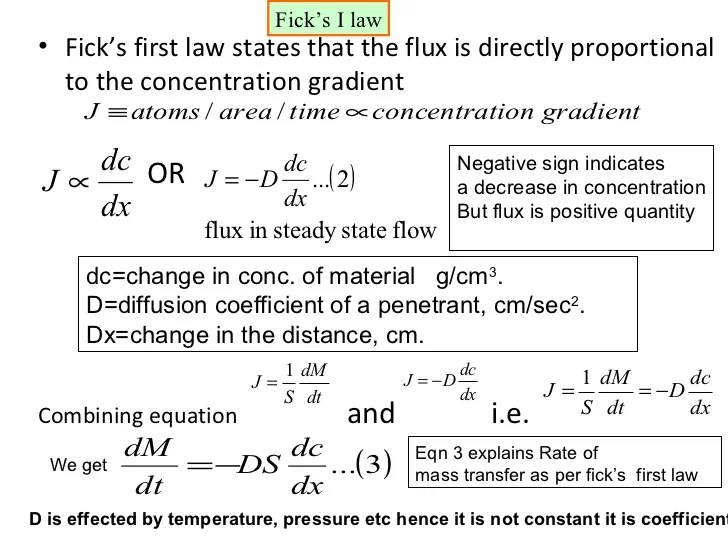
Fick's First Law Of Diffusion:
Under steady state condition calculate the diffusion flux of carbon through the plate, if the concentration of carbon at position of 5 mm and 10 mm beneath the carburizing surface are 1.2 kg/m3 and 0.8 kg/m3, respectively. This is also referred to as the movement of a substance down a concentration gradient. Flux is directly proportional to gradient.
Describe Fick's First Law Of Diffusion.
Fick's first law states the two simple facts related to transfer mechanisms: Fick’s law is applicable for two miscible liquids when they are brought in contact and diffusion takes place at a macroscopic level. Define a new parameter, the diffusion coefficient, d:
A Simple Explanation Of Fick's First Law Of Diffusion.
So, finally, a mathematical equivalent to fick's law, in both a continuous (calculus) and discrete version. Ficks second law cylindrical coordinates. A diffusion process that obeys fick's laws is called normal or fickian diffusion;
Fick's Law Of Diffusion — Fick S Laws Of Diffusion Describe Diffusion And Can Be Used To Solve For The Diffusion Coefficient D.
This quantity is called the diffusion flux with units of. What is fick’s law equation? Diffusion of chemicals in a.
Fick's Laws Of Diffusion Describe Diffusion And Were Derived By Adolf Fick In 1855.
In last two lectures, we learned the thermodynamics, concerned mainly with stable or equilibrium systems. Learn about fick's first law, discover its applications, and learn about the equation and calculations. They can be used to solve for the diffusion coefficient dficks first law can be used to derive his second law which in turn is identical to the diffusion equation.
Comments
Post a Comment